A sodium-vapor lamp is a gas-discharge lamp that uses sodium in an excited state to produce light at a characteristic wavelength.
Two varieties of such lamps exist: low pressure and high pressure. Low-pressure sodium lamps are highly efficient electrical light sources, but their yellow light restricts applications to outdoor lighting, such as street lamps, where they are widely used. High-pressure sodium lamps emit a broader spectrum of light than the low-pressure lamps, but they still have poorer color rendering than other types of lamps.
Low-pressure sodium lamps only give monochromatic yellow light and so inhibit color vision at night.
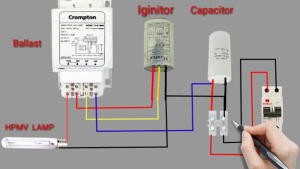
Sodium vapour lamps require external components in order to operate. The ballast is for limiting the current, the igniter is for igniting the lamp and the capacitor is for improving the flow of electricity into the fixture which isn’t needed in most fixtures.
These days the sodium vapour lamp is becoming less common as time goes on because they are being replaced with more energy efficient LED lights.
The first sodium vapour lamp was invented in 1920 by Arthur H Comptom at westinghouse. The first lamp was a round bulb with two electrodes on each side.
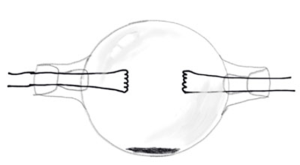
The solid sodium metal remained on the bottom center of the bulb. When heated up the metal would vaporize and the lamp would glow yellow. The lamp had to be designed in a sphere because after the metal cooled when the lamp was turned off, the sodium has a property of migrating to the coolest part of the bulb where it solidifies. A tube design would be more particle, similar to the neon lamp which had already been developed by 1920 but it was found that the sodium would migrate to the outer ends of the tube, and there the sodium would destroy the electrodes as well as not get hot enough to vapourize.



